- Nucleation
- Continuous Crystallization and Novartis-MIT Center for Continuous Manufacturing
- Separation Methods
Nucleation
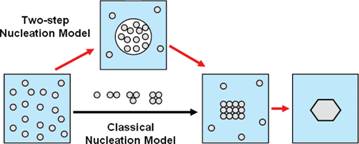
In crystallization from solution, nucleation plays a decisive role in determining the crystal form, size distribution, shape and purity and therefore understanding the fundamentals of nucleation is crucial to achieve control over these properties. Classical nucleation theory is widely applied to solution crystallization due to its simplicity; however, its shortcomings suggest that nucleation of crystalline solids from solution does not proceed via the classical pathway, but rather follows more complex routes. In the last decade, numerous studies including those reported by our group have suggested an alternative mechanism of crystal nucleation from solution called the two-step mechanism.
Areas of focus:
-
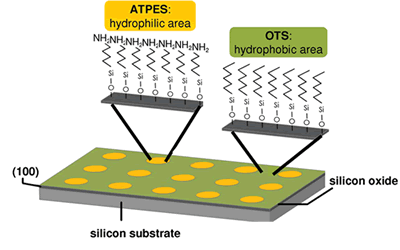
Heterogeneous nucleation
- Nucleation on polymeric surfaces
- Nucleation on crystalline surfaces
- Development of processes for both of the above for the continuous crystallization of active pharmaceutical ingredients
- Contact secondary nucleation: studying the mechanism of contact secondary nucleation and applying it in mini-tube / microfluidic systems
- Nucleation in micro/nano-pores to manufacture nano-sized crystals
Continuous Crystallization and Novartis-MIT Center for Continuous Manufacturing
The Novartis-MIT Center for Continuous Manufacturing is a 10-year research collaboration aimed at transforming pharmaceutical production. Combining the industrial expertise of Novartis with MIT’s scientific and technological leadership, the Center develops new technologies to replace the pharmaceutical industry’s conventional batch-based system with a continuous manufacturing process. Continuous manufacturing will benefit patients, healthcare providers, and the pharmaceutical industry by:
- Accelerating the introduction of new drugs through efficient production processes
- Requiring the use of smaller production facilities with lower building and capital costs
- Minimizing waste, energy consumption, and raw material use
- Monitoring drug quality on a continuous basis rather than through post-production batch-based testing
- Enhancing process reliability and flexibility to respond to market needs
Our group, in cooperation with the Novartis-MIT Center is exploring the use of various types of continuous crystallization for pharmaceutical intermediates and final crystallization of active pharmaceutical ingredients (APIs). Issues related to purity, polymorphism, crystal size distribution, crystal shape, and scale-up and recycle are being studied.
Areas of focus:
- Process design of continuous crystallization to increase yield of active pharmaceutical ingredients
- Process design of continuous crystallization to enhance product purity
- Novel Crystallizer Designs
Separation Methods
Our group is developing various separation methods for pharmaceutical compounds.
Areas of focus:
- Ionic liquids
- Co-crystals: co-crystals are multiple-component crystal systems that coexist through hydrogen bonds or non-covalent interactions. The reactants are solids at ambient conditions. The development of co-crystals of active pharmaceutical ingredients is of great current interest as a means of increasing the solubility of poorly soluble drugs. However, co-crystals can also be used as a means of separation. For example, a co-crystal could be formed with an impurity but not with the desired component. If the solubility of the co-crystal is very low, it could be possible to crystallize out the impurity (as the co-crystal) while leaving the desired component in solution. This can be advantageous for pharmaceutical intermediates by eliminating their crystallization. Our group is currently evaluating various strategies for employing co-crystals for separations.
- Self-assembled monolayers (SAMs) and chiral separation: SAMs are ordered molecular assemblies that are formed spontaneously by the adsorption of a surfactant with a specific affinity of its headgroup to a substrate. A SAM molecule consists of a headgroup, chain or backbone, and endgroup. A SAM system is typically defined by the headgroup-substrate pair such as thiol SAMs on gold substrates and silane-based SAMs on SiO2. One application of SAM is chiral separation. Chiral drugs are a subgroup of drug substances that contain one or more chiral centers. The pure enantiomer is preferred over the racemate in mnay marketed dosage forms because of safety and efficacy. In our research, we used chiral self-assembled monolayers on gold as resolving auxiliaries in the crystallization of chirality-specific drugs.